Heart failure with preserved ejection fraction (HFpEF), also known as diastolic heart failure, is a type of heart failure characterized by the inability of the heart to fill with and adequately pump blood while the ejection fraction—the percentage of blood pumped out of the heart during each contraction—is normal or near-normal. In HFpEF, the main problem lies in the impaired ability of the heart to relax and fill with blood during the resting phase between heartbeats (diastole).
Ashley Davidoff
TheCommonVein.net
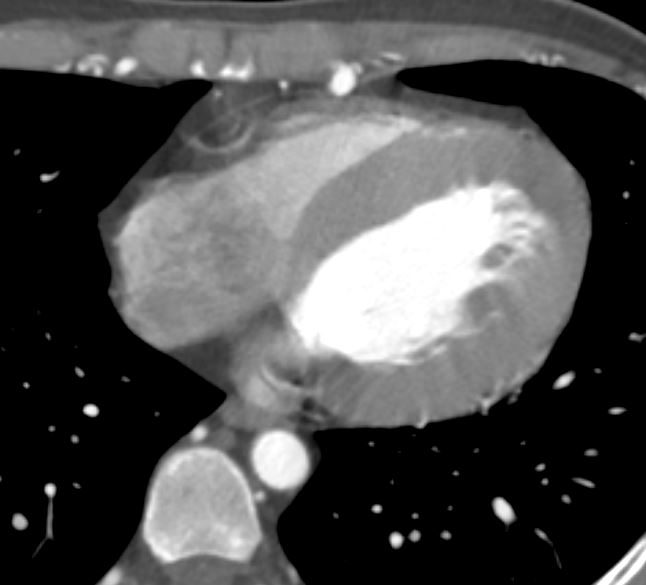
The LV in systole shows concentric thickening and this case demonstrates LVH
Note the mitral valve is closed
Ashley Davidoff MD TheCommonVein.net wall-thickness-002
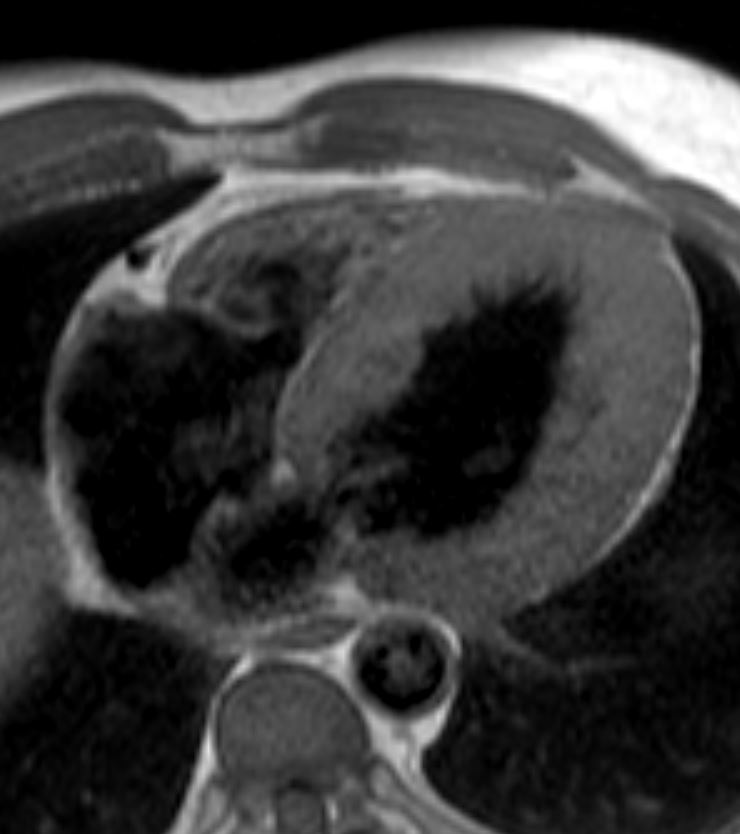
Concentric Hypertrophy
Anomalous RCA from LCA
Ashley Davidoff
thecommonvein.net
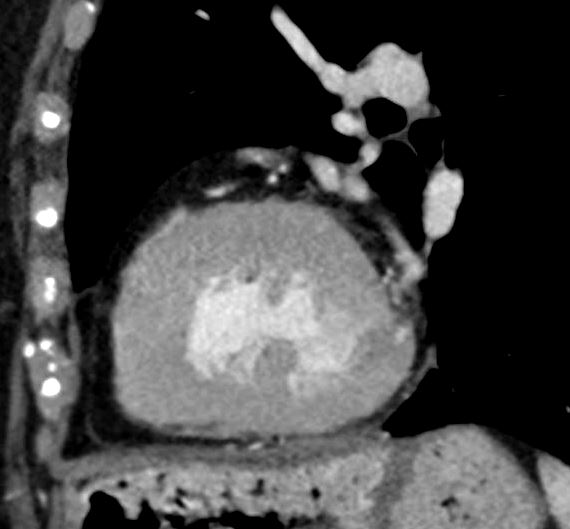
LVH-003b
tags concentric hypertrophy LVH, hypertension
Ashley Davidoff MD
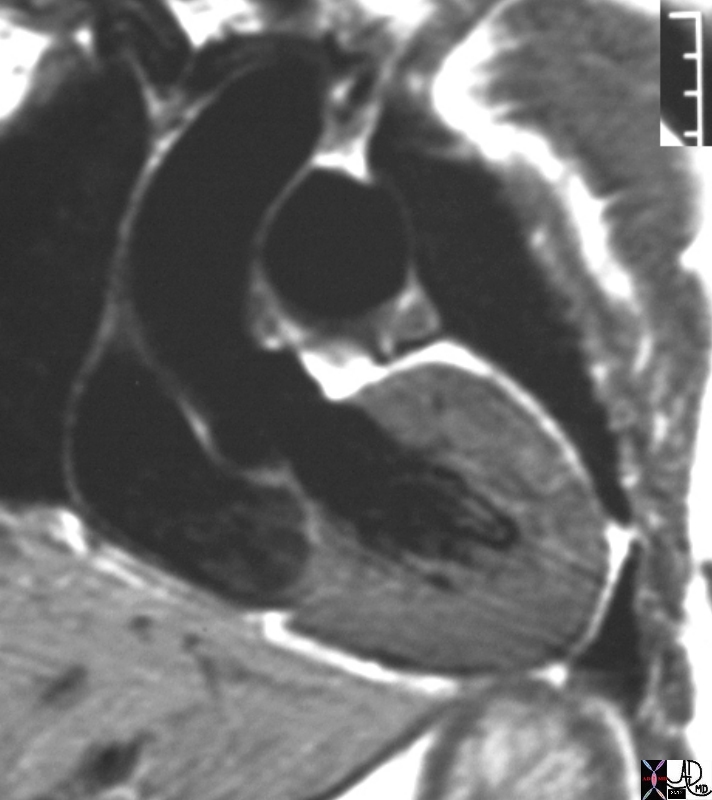
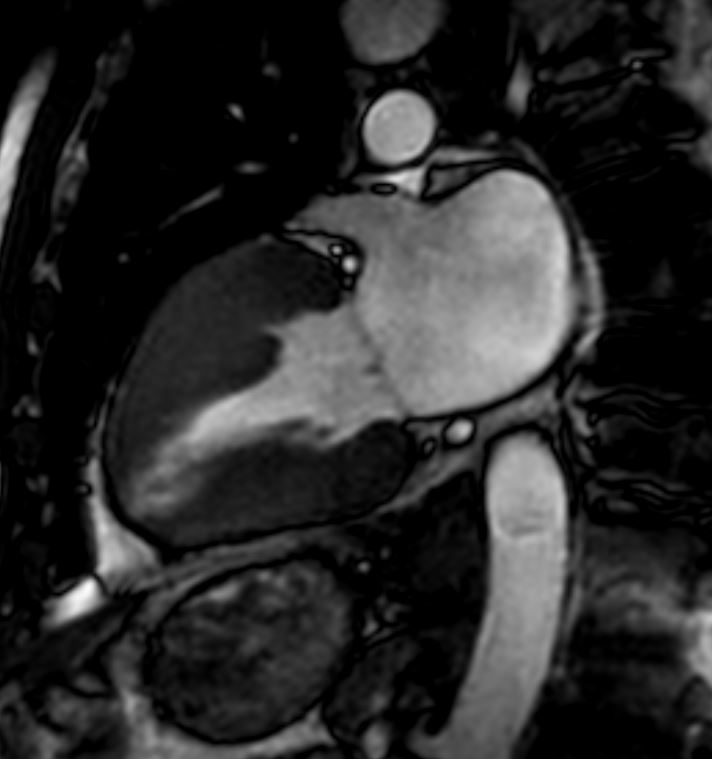
# heart LV left ventricle aorta coarctation left ventricular hypertrophy fx LVH concentric hypertrophy dx coarctation imaging radiology T1 weighted MRI Courtesy Ashley Davidoff MD TheCommonVein.net 16906.800
Normal Thickness of the LV
Ashley Davidoff MD
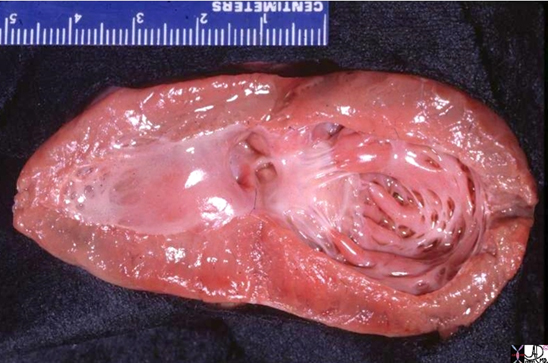
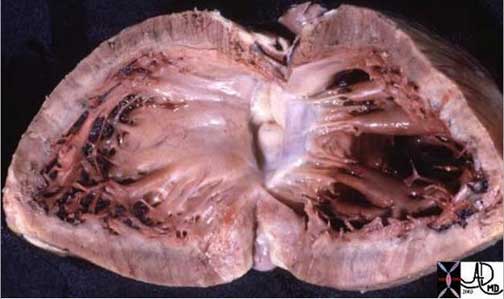
Shining Glistening Endothelium of the Left Ventricle
The left ventricle that has been opened along the ventricular septum shows the septal wall to your left and the free wall with the papillary muscles to your right. Note the glistening endothelium which has a whitish sheen. The myocardium lies directly under the endocardium. It is lighter red, muscular and rubbery. Note also the two sets of papillary muscles, the fibrous nature of the valves and the fibrous continuity of the mitral valve with the aortic valve. A VSD is noted on the superior aspect of the septum. Courtesy of: Ashley Davidoff, M.D.
08345b.8
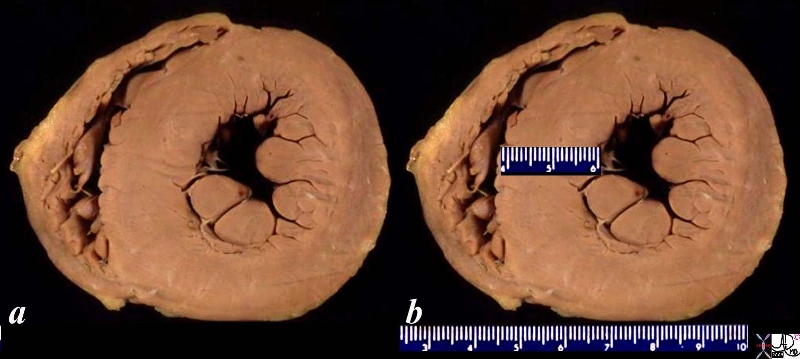
08028b06 heart cardiac LV left ventricle papillary muscles RV right ventricle LVH left ventricular hypertrophy thickened LV concentric hypertrophy interventricular septum thickened enlarged gross pathology Courtesy Ashley Davidoff MD
08028b06 heart cardiac LV left ventricle papillary muscles RV right ventricle LVH left ventricular hypertrophy thickened LV concentric hypertrophy interventricular septum thickened enlarged gross pathology Courtesy Ashley Davidoff MD
LVH-004
Ashley Davidoff MD
Cardiac amyloidosis is a condition where abnormal proteins called amyloid fibrils build up in the heart tissue, leading to heart dysfunction. There are different types of amyloidosis, and one specific type associated with heart failure with preserved ejection fraction (HFpEF) is called transthyretin amyloidosis (ATTR).
ATTR amyloidosis can be further classified into two main subtypes based on the source of the amyloid fibrils:
- Wild-type ATTR (ATTRwt): In this subtype, the amyloid fibrils are derived from the normal or wild-type transthyretin protein. Wild-type ATTR primarily affects older individuals and is typically associated with HFpEF. The exact mechanisms underlying the development of HFpEF in wild-type ATTR are not fully understood, but the infiltration of amyloid deposits in the heart muscle can result in diastolic dysfunction and impaired relaxation, leading to symptoms of heart failure.
- Hereditary ATTR (ATTRm): This subtype of ATTR amyloidosis is caused by genetic mutations in the transthyretin gene. The mutated transthyretin protein forms amyloid fibrils that can accumulate in various organs, including the heart. While hereditary ATTR can also cause heart failure, it is more commonly associated with heart failure with reduced ejection fraction (HFrEF), where the ejection fraction is significantly decreased.
It’s worth noting that while cardiac amyloidosis, particularly ATTR amyloidosis, can contribute to HFpEF, there are various other causes of HFpEF unrelated to amyloidosis. These causes can include hypertension, obesity, diabetes, coronary artery disease, and other structural or functional abnormalities of the heart.
Diagnosing the specific type of amyloidosis causing HFpEF requires a comprehensive evaluation by a healthcare professional, including a thorough medical history, physical examination, imaging tests (such as echocardiography and cardiac MRI), and sometimes a biopsy to confirm the presence of amyloid deposits and determine the specific subtype. Treatment approaches for amyloidosis-associated HFpEF may involve managing symptoms, controlling underlying conditions, and, in some cases, targeting the specific amyloid protein with emerging therapies.
Ashley Davidoff TheCommonVein.net
Ashley Davidoff TheCommonVein.net
Ashley Davidoff TheCommonVein.net

Ashley Davidoff TheCommonVein.net

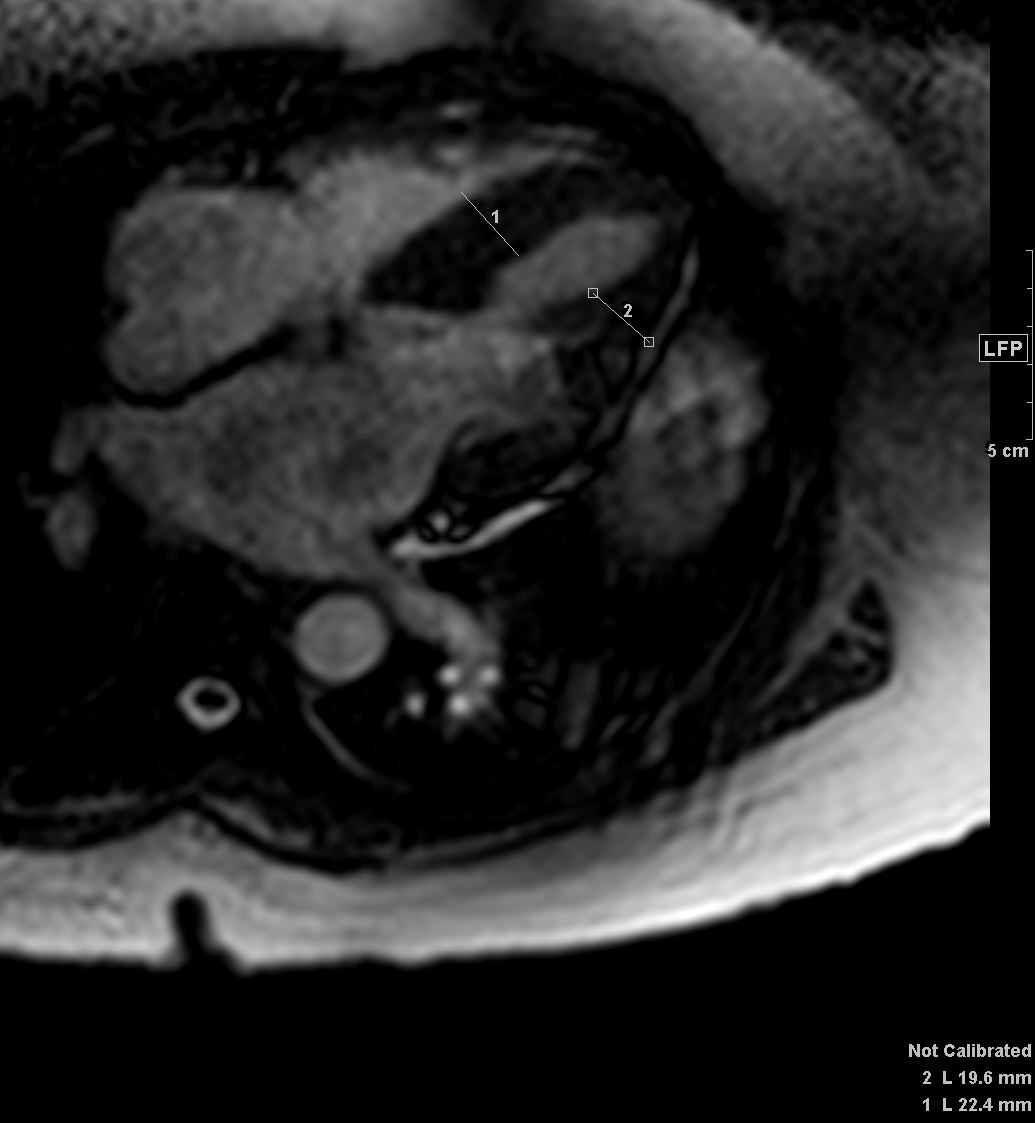
Late gadolinium enhancement in amyloidosis. Because certain artefacts regarding acquisition timing may occur when acquiring late gadolinium enhancement (LGE), the preferred technique to do LGE in amyloidosis is phase-sensitive inversion recovery (PSIR). PSIR allows to characterise better the segments that have gadolinium enhancement. The characteristic pattern is ‘global endocardial’, which also has been described as having transmural and ‘patchy’ characteristics. This case illustrated above looks mostly transmural and patchy. Note biventricular thickening
Agha, A et al Role of cardiovascular imaging for the diagnosis and prognosis of cardiac amyloidosis Open Heart BMJ
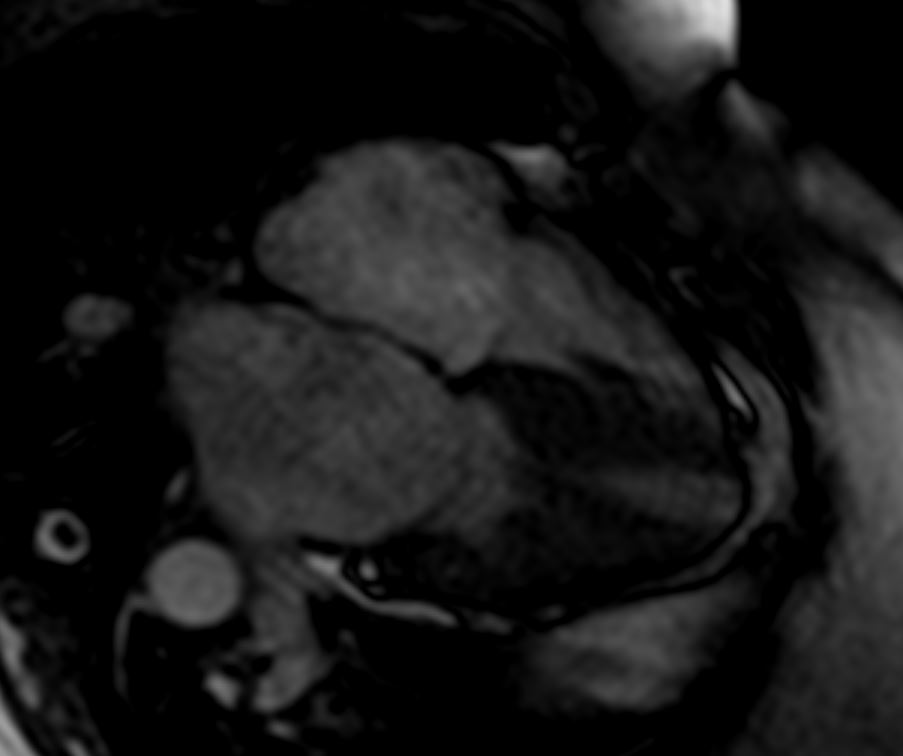
82 year old female
with diastolic heart failure
4 chamber view shows left ventricular hypertrophy and left effusion and mild enlargement of the left atrium
Ashley Davidoff MD
heart-00066
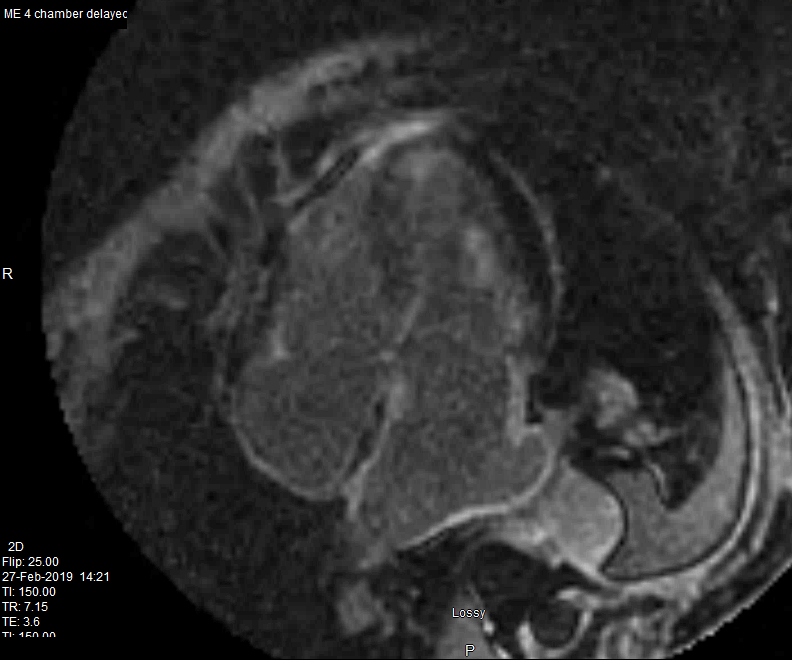
82 year old female
with diastolic heart failure
Delayed enhancement is noted in the walls of both atria and a resulting zebra appearance (white black white) of the interatrial septum. LGE also noted in the
papillary muscle in the subendocardial regions of the LV and RV
Ashley Davidoff MD
heart-00064
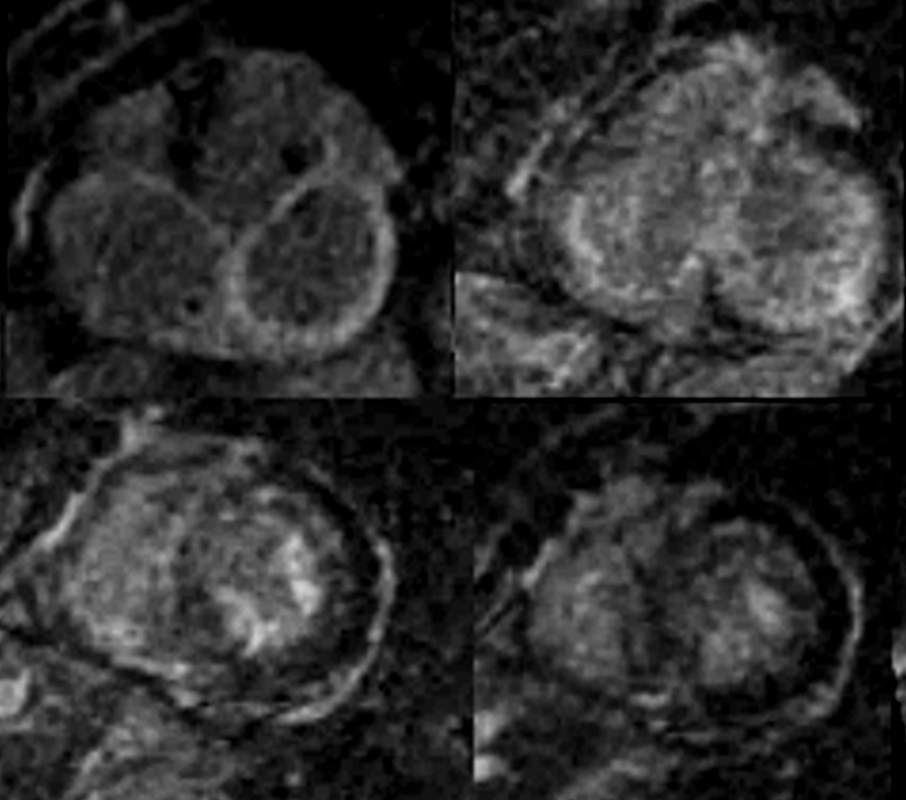
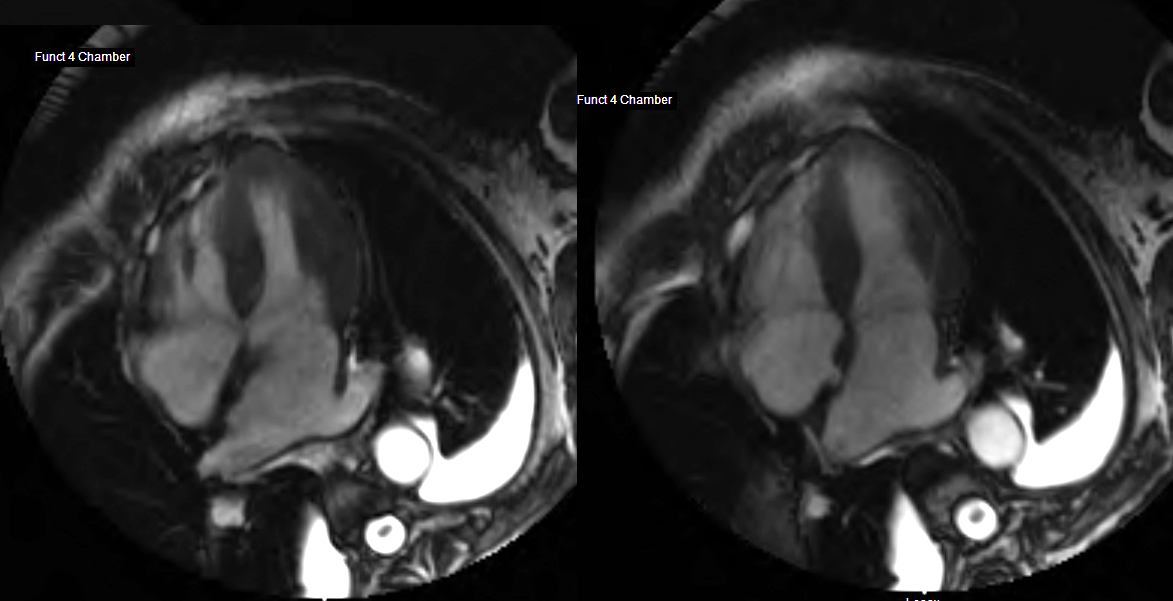
MRI in Short Axis projection following Gd injection
82 year old female with history of diastolic heart failure
Top left – Atrial wall delayed enhancement
Top right – LGE in subendocardial regions of LV and RV
Bottom left – LGE in papillary muscles and subendocardial regions
Bottom right LGE in papillary muscles and subendocardial regions
Courtesy Ashley Davidoff MD
heart-00063
Here are some key points about heart failure with preserved ejection fraction:
- Ejection Fraction: Ejection fraction (EF) is a measurement of the percentage of blood pumped out of the left ventricle with each heartbeat. In HFpEF, the ejection fraction is typically normal or mildly reduced, usually above 50%, indicating that the heart’s ability to contract and pump blood is preserved.
- Diastolic Dysfunction: The primary issue in HFpEF is impaired diastolic function, which refers to the heart’s ability to relax and fill with blood during diastole. The stiffness of the heart muscle (ventricular stiffness) and reduced ventricular compliance hinder proper filling, resulting in elevated pressures within the heart and lungs.
- Symptoms: The symptoms of HFpEF are similar to those of other types of heart failure and may include shortness of breath, fatigue, exercise intolerance, fluid retention, swollen ankles, and a reduced ability to engage in physical activities. However, some individuals with HFpEF may remain asymptomatic or have atypical symptoms.
- Risk Factors: HFpEF is commonly associated with certain risk factors, including older age, hypertension (high blood pressure), obesity, diabetes, coronary artery disease, atrial fibrillation, and chronic kidney disease. These conditions can contribute to the development of structural and functional changes in the heart Elderly with amyloidosis
- Diagnosis: The diagnosis of HFpEF involves a comprehensive evaluation, including a medical history review, physical examination, echocardiography (ultrasound of the heart), and other tests to assess heart function, identify potential underlying causes, and rule out other conditions with similar symptoms.
- Treatment: The management of HFpEF focuses on controlling symptoms, preventing exacerbations, and addressing underlying causes. This typically involves lifestyle modifications such as regular exercise, dietary changes (e.g., reducing salt intake), managing comorbidities (e.g., hypertension, diabetes), and medications to control blood pressure and heart rate. In some cases, medications that reduce symptoms and improve outcomes in heart failure with reduced ejection fraction (HFrEF) may also be used. Additionally, close monitoring and regular follow-up with a healthcare provider are crucial.
- Prognosis: HFpEF has been considered more challenging to treat compared to HFrEF, and there are currently no specific therapies that have shown consistent benefits in reducing mortality or hospitalizations. The prognosis of HFpEF can vary, but it is generally associated with a substantial burden of symptoms and an increased risk of hospitalization and cardiovascular events.